62+ calculate the energy of a photon of electromagnetic radiation
Web The Photon energy formula is given by E h c λ Where E photon energy h Plancks constant 66261034 Js c speed of the light and λ wavelength of the light. Identify if the wavelength or the frequency of the photon is given and what the value is.

Solved Calculate The Energy Of A Photon Of Electromagnetic Chegg Com
Web Calculate the energy of a photon of electromagnetic radiation ateach of the following wavelengths6328 wavelength of red lightfrom helium-neon laser This problem has been solved.
. 8356 MHz common frequency used for cell phone communication. 1033 MHz typical frequency for FM radio broadcasting. Web Calculate the energy of a photon of electromagnetic radiation at each of the frequencies indicated in Problem 40.
E h c λ 6 626 10 34 J s 3 10 8 m s 724 10 9 m 27 4 _ 558 10 19 J p h o t o n. 1062 MHz typical frequency for FM radio broadcasting 1065 kHz typical frequency for AM radio broadcasting assume four significant figures. An equation that relates energy and frequency is.
So this online energy of a photon calculator to determine the results in different smallest units. Web The energy of a photon is a smaller quantity as the Plancks constant has a small number. Calculate the energy of a photon of electromagnetic radiation at each of the following frequencies.
Youll get a detailed solution from a subject. Web This calculator computes the energy of a photon from its vacuum wavelength lambda λ frequency nu ν or wavenumber kappa κ. Web Calculate the energy of a photon of electromagnetic radiation with a wavelength of 4629 nm.
E_ p hnu dfrac hc lambda hckappa E p hν λhc hcκ. We only know the frequency of the photon the frequency is 541014 Hz 54 10 14. 6328 nm wavelength of red light from.
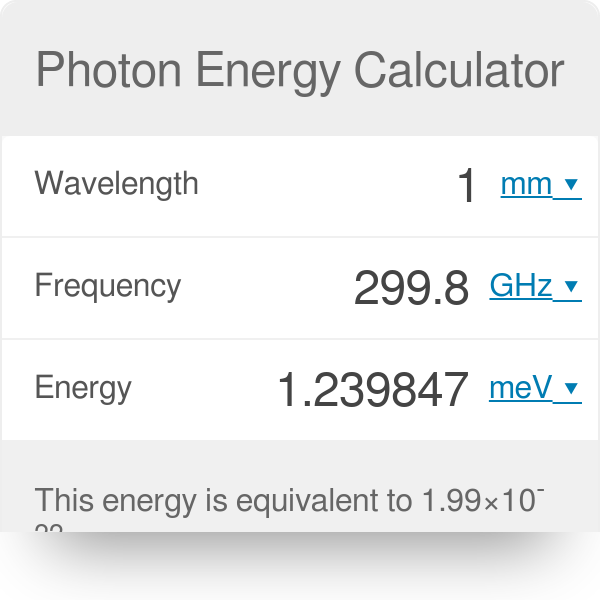
Web So first lets calculate the energy of one photon. Web Energy of a photon formula is E h x c E 66261 x 10 -34 x 299 792 4583 E 04132823 meV Frequency f h x E f 66261 x 10 -34 x 04132823 9993 GHz. Where h approx 6626cdot 10 -34 h 6626 1034 is the Planck constant and c c is the speed of light in vacuum.
Web Calculate the energy of a photon of electromagnetic radiation at each of the following frequencies. E hnu E energy in Joules J h Plancks constant 6626xx10-34Js nu frequency Hz or. Web We need to determine the energy for a single photon of radiation that has a frequency that measures 396 times 10 to the 15th power inverse seconds.
Also to further explore the relationship between frequency wavelength you can use this online calculator. 1070 kHz typical frequency for AM radio broadcasting assume four significant figures c. The underlined number is to keep track of three significant figures which we will round off the final answer to.
Our first step is to recall that. 1002 MHz typical frequency for FM radio broadcasting b. 10 Report your answer in scientific notation using the provided boxes.
Web Calculate the energy of a photon of electromagnetic radiation at each of the wavelengths indicated in Problem 39. The photon energy is.
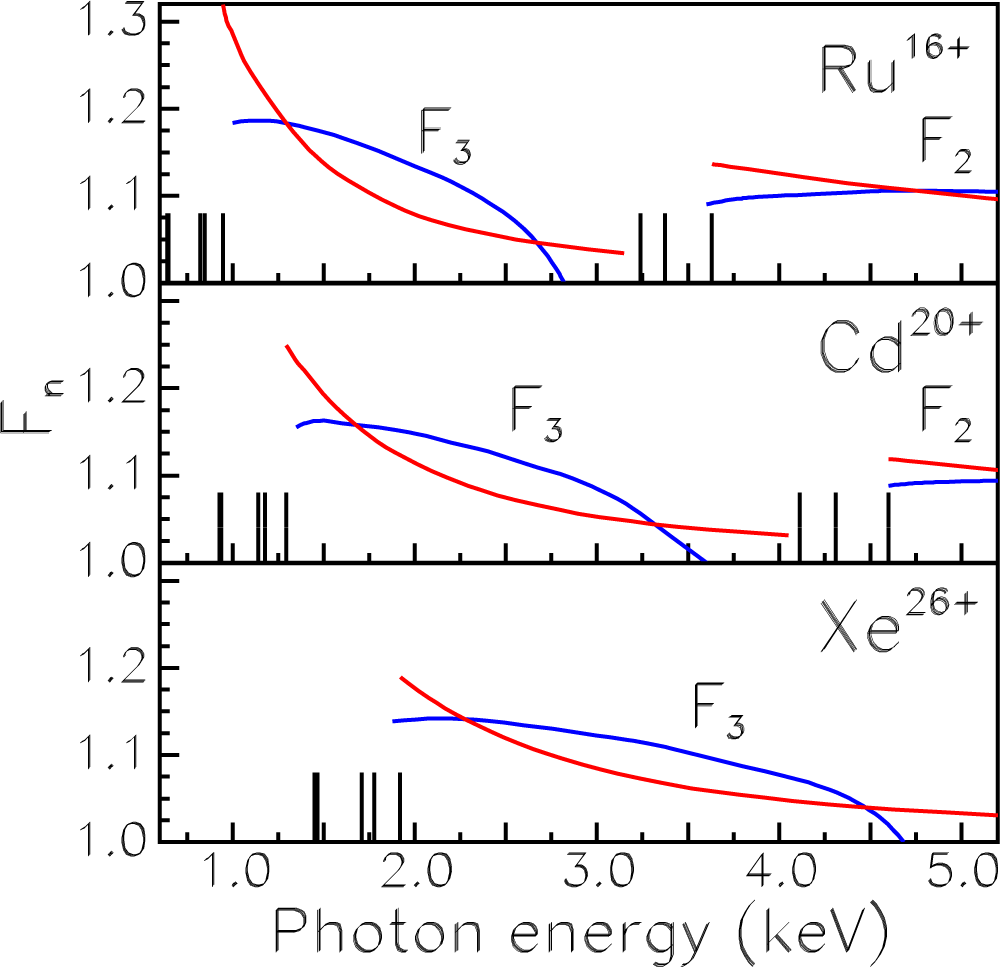
Atoms Free Full Text Radiative Recombination And Photoionization Data For Tungsten Ions Electron Structure Of Ions In Plasmas
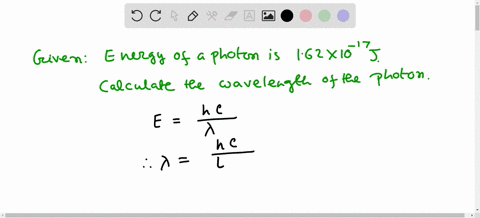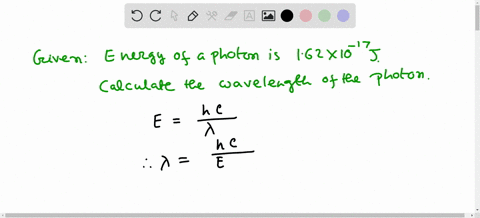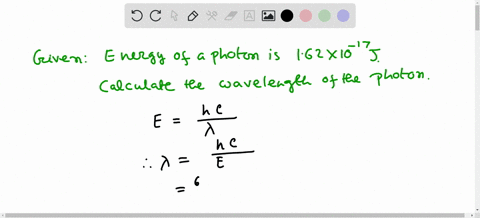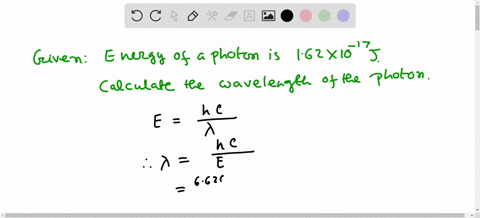
Solved Calculate The Wavelength Of Electromagnetic Radiation With An Energy Per Photon Of 3 00 X 10 19 589 Nm B 1 36 X 1032 Nm 358 Nm 6 62 X 10 7 Nm 662 Nm

Energy Of Photon Is 3ev Find The Frequency And Wavelength Of Radiation I Need Compete Answer Brainly In

Calculate The Wavelength Of Photon Having Energy 5ev Youtube

A Photon Of Radiation Of Wavelengh 600 Nm Has An Energy E The Wavelength Of Photon Of Radiation Having Brainly In

What Is The Energy Of Photon Whose Wavelength Is 6840 A
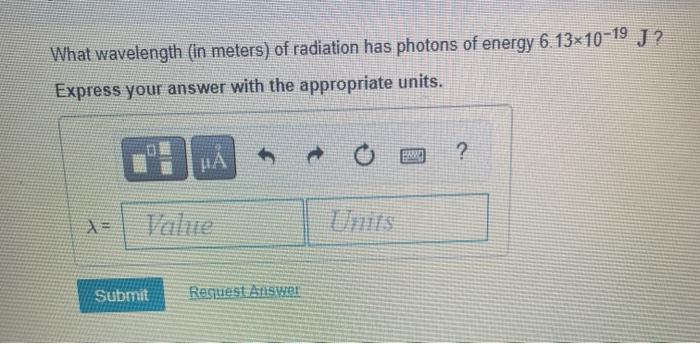
Solved Calculate The Energy In Joules Of A Photon Of Chegg Com

Find Energy Of Each Of The Photons Which A Correspond To Light Of Frequency 3xx10 15 Hz B Youtube
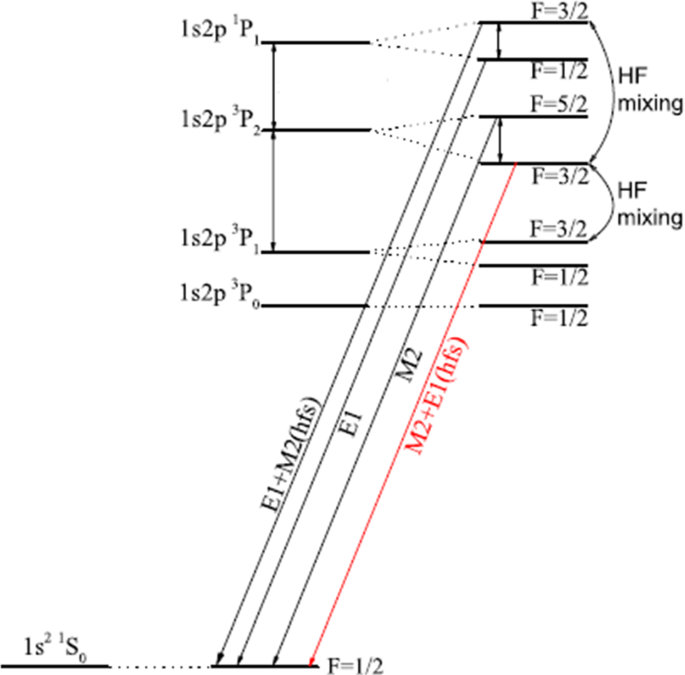
Hyperfine Induced Depolarization Effect In The X Ray Line Emission Mathrm 1s2p 3p 2 Rightarrow 1s 2 1s 0 Of He Like Ions Springerlink

Pdf Caqs Ebook Alex Milliron Academia Edu

What Is The Wavelength Of The Radiation With Photon Energy Which Is The Mean Value Of Photon Energies Of Radiations With Wavelength Lamda1 4000 A And Lamda2 6000 A

Photon Energies And The Electromagnetic Spectrum Physics
First Document Gsi
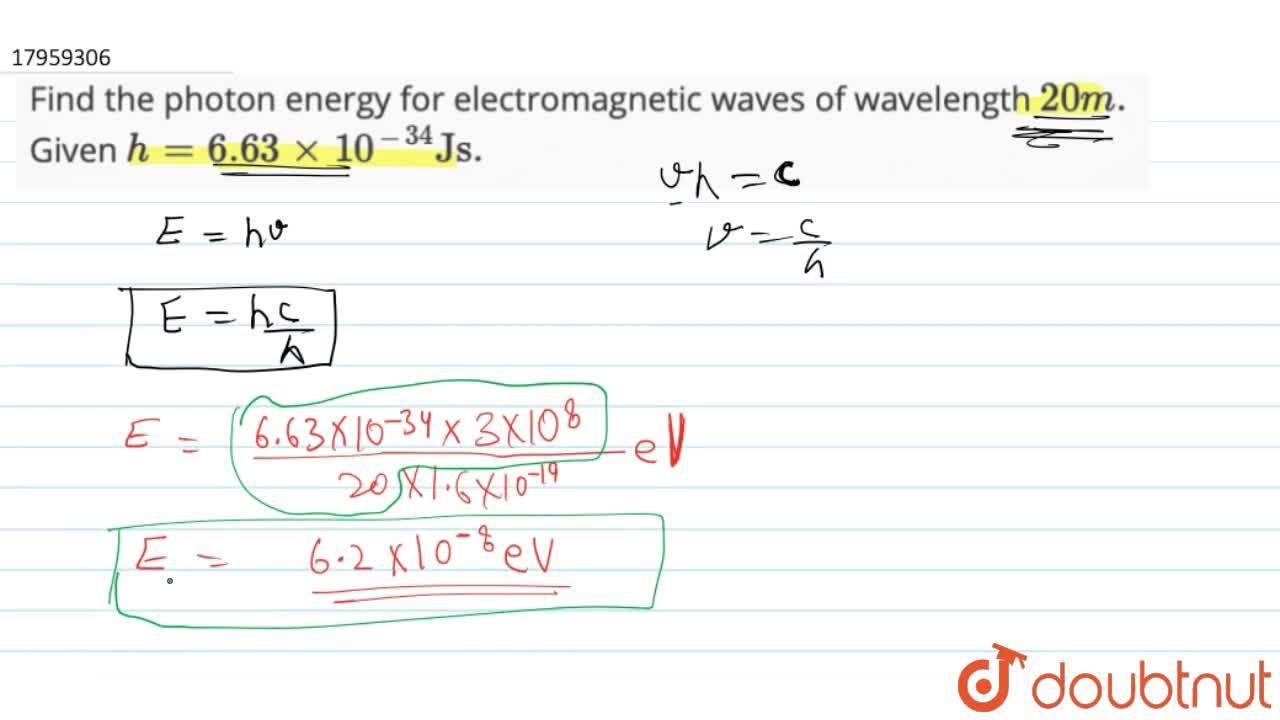
Find The Photon Energy For Electromagnetic Waves Of Wavelength 20m Given H 6 63xx10 34 Js
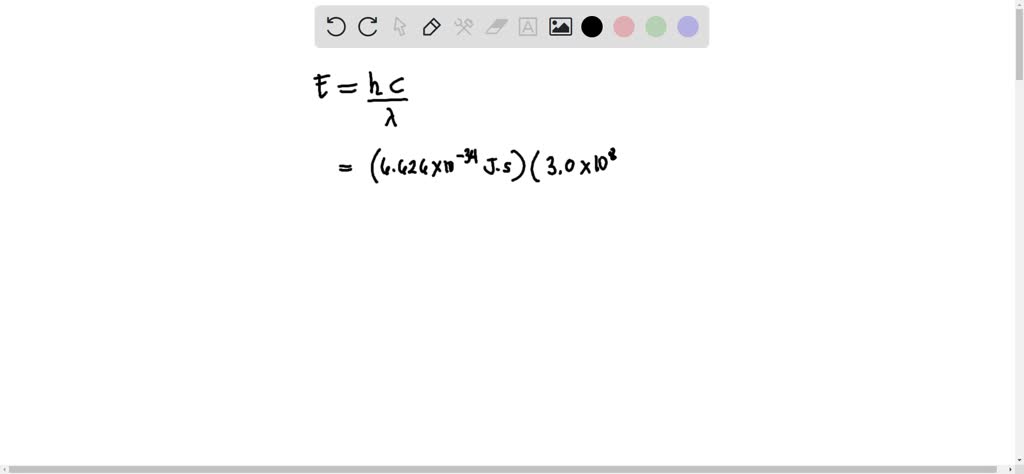
Solved Calculate The Wavelength Of Electromagnetic Radiation With An Energy Per Photon Of 3 00 X 10 19 589 Nm B 1 36 X 1032 Nm 358 Nm 6 62 X 10 7 Nm 662 Nm

Find Energy Of Each Of The Photons Which I Correspond To Light Of Frequency 3 10 15hz Ii Have Wavelength Of 0 50 A

Pdf Effects Of Qed And Beyond From The Atomic Binding Energy