18+ Calculate Delta G For The Reaction At 298 K
This change of a few words more than doubled the pension payout from 27 million to an estimated 58 million but these numbers did not appear on the SEC-required executive compensation tables or in the annual report footnotes. Insolubility is the opposite property the inability of the solute to form such a solution.

For The Reaction At 298 K A G B G Harrd G C G Deltah 29 8 Kcal And Deltas 100calk 1 Calculate The Value Of Equilibrium Constant
California voters have now received their mail ballots and the November 8 general election has entered its final stage.

. Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference as a measurable and quantitative phenomenon and identifiable chemical change with the potential difference as an outcome of a particular chemical change or vice versaThese reactions involve electrons moving via an electronically-conducting phase. For each solution convert the mass of KNO3 per 500 g water into an equivalent mass of KNO3 per 100 g of water. The rate of the chemical reaction doubles for an increase of 10 K in absolute temperature from 298 K.
For a similar process at constant temperature and volume the change in Helmholtz free energy must be negative. A 298 K temperature. A solution containing 30g of non-volatile solute exactly in 90 g of water has a vapor pressure of 28 kPa at 298 K.
Thus a negative value of the change in free energy G or A is a necessary condition for a process to be spontaneous. Salivation that is usually similar to. Each gas has a pressure of one bar.
The numbers were revealed only because a newspaper covering the story hired an actuary to calculate the new basis. Therefore even at absolute zero atoms and molecules retain some vibrational motionApart from atoms and molecules the. Microsoft describes the CMAs concerns as misplaced and says that.
The latest Lifestyle Daily Life news tips opinion and advice from The Sydney Morning Herald covering life and relationships beauty fashion health wellbeing. Pb l 05 O 2 g PbO s For Lead. At 25 what is the value of delta G.
Microsoft pleaded for its deal on the day of the Phase 2 decision last month but now the gloves are well and truly off. Molar mass of the solute. T m 1158 K Δ H m 27.
Further 18g of water is then added to the solution and the new of vapor pressure becomes 29 kPa at 298 K. 5 F6 Solution 1 2 3 Temperature C 4 CD A 225 z 501 664 766 KNO3 Solubility Temperature g100 g H2O C S PrtScn T 18 F7 Home F8 y 69F End F9 Pg. ASCII characters only characters found on a standard US keyboard.
6 to 30 characters long. CDC recommends universal indoor masking for all teachers staff students and visitors to K-12 schools regardless of vaccination status. The pressurevolume term expresses.
Unlike in classical mechanics quantum systems constantly fluctuate in their lowest energy state as described by the Heisenberg uncertainty principle. This is the most useful form of the second law of thermodynamics in chemistry where free-energy. According to the second law of thermodynamics for systems reacting at fixed temperature and pressure without input of non-Pressure Volume PV work there is a general natural tendency to achieve a minimum of the Gibbs free energy.
A reaction has delta H 563 kJ and delta S 132 JK. Calculate Δ H 1000 and Δ S 1000 for the following reaction. Children should return to full-time in-person learning in the fall with layered prevention.
Amid rising prices and economic uncertaintyas well as deep partisan divisions over social and political issuesCalifornians are processing a great deal of information to help them choose state constitutional officers and. Here we report the results of an international collaboration to produce. To drive such a reaction we need to have input of free energy ie the reaction is endergonic The factors affect Delta G of a reaction assume Delta H and Delta S are independent of temperature.
Spontaneity of a Reaction Delta G - nFE_cell. A quantitative measure of the favorability of a given reaction under these conditions is the change ΔG sometimes written delta G or dG. Food is paired with a previously neutral stimulus eg.
Must contain at least 4 different symbols. Enthalpy ˈ ɛ n θ əl p i a property of a thermodynamic system is the sum of the systems internal energy and the product of its pressure and volume. The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a saturated solution one in which no more solute can.
Delta G 0. A triangleIt also refers to the learning process that results from this pairing through which the neutral stimulus comes to elicit a response eg. Given that Mass of non-volatile solute 30 g.
Give the calculator answer. In 2017 the US. T m 600 K Δ H m 4 810 J mol CP P s 236 975 E 3 T J mol CP P I 324 310 E 3 T J mol Δ H 298 0 J mol S 298 649 J K 298 600 K 600 1200 K For Lead Oxide.
Solid K 2 CO 3 514 g 372 mmol was added to the reaction flask and left to stir at 70 C for 15 h until TLC analysis 21 HexEtOAc vv indicated the complete conversion to the product. Because cannabis is illegal in most countries clinical research presents a challenge and there is limited evidence from which to draw conclusions. Or dG 0.
In chemistry and thermodynamics the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements in their reference state with all substances in their standard statesThe standard pressure value p 10 5 Pa 100 kPa 1 bar is. None of these g. On the other hand cannot be used to calculate the value of molar conductivity of a weak electrolyte at infinite dilution since the curve becomes practically parallel to the y-axis as concentration approaches zero.
The human genome holds an extraordinary trove of information about human development physiology medicine and evolution. National Academies of Sciences Engineering and Medicine issued a report summarizing much of the published literature on health effects of. Reaction is not spontaneous and the process proceeds spontaneously in the reserve direction.
It is a state function used in many measurements in chemical biological and physical systems at a constant pressure which is conveniently provided by the large ambient atmosphere. The formula that can be used to solve the question is. Microsoft has responded to a list of concerns regarding its ongoing 68bn attempt to buy Activision Blizzard as raised by the UKs Competition and Markets Authority CMA and come up with an.
Classical conditioning also known as Pavlovian or respondent conditioning is a behavioral procedure in which a biologically potent stimulus eg. Given new evidence on the B16172 Delta variant CDC has updated the guidance for fully vaccinated people. The long-term effects of cannabis have been the subject of ongoing debate.
In chemistry solubility is the ability of a substance the solute to form a solution with another substance the solvent. Zero-point energy ZPE is the lowest possible energy that a quantum mechanical system may have. Let molar mass of the same.

Energetic Insight Into The Formation Of Solids From Aluminum Polyoxocations Reusser 2015 Angewandte Chemie International Edition Wiley Online Library
Mar Bjmp Org

The Gibbs Free Energy Post 16 Thermodynamics Tutorials Resource Rsc Education

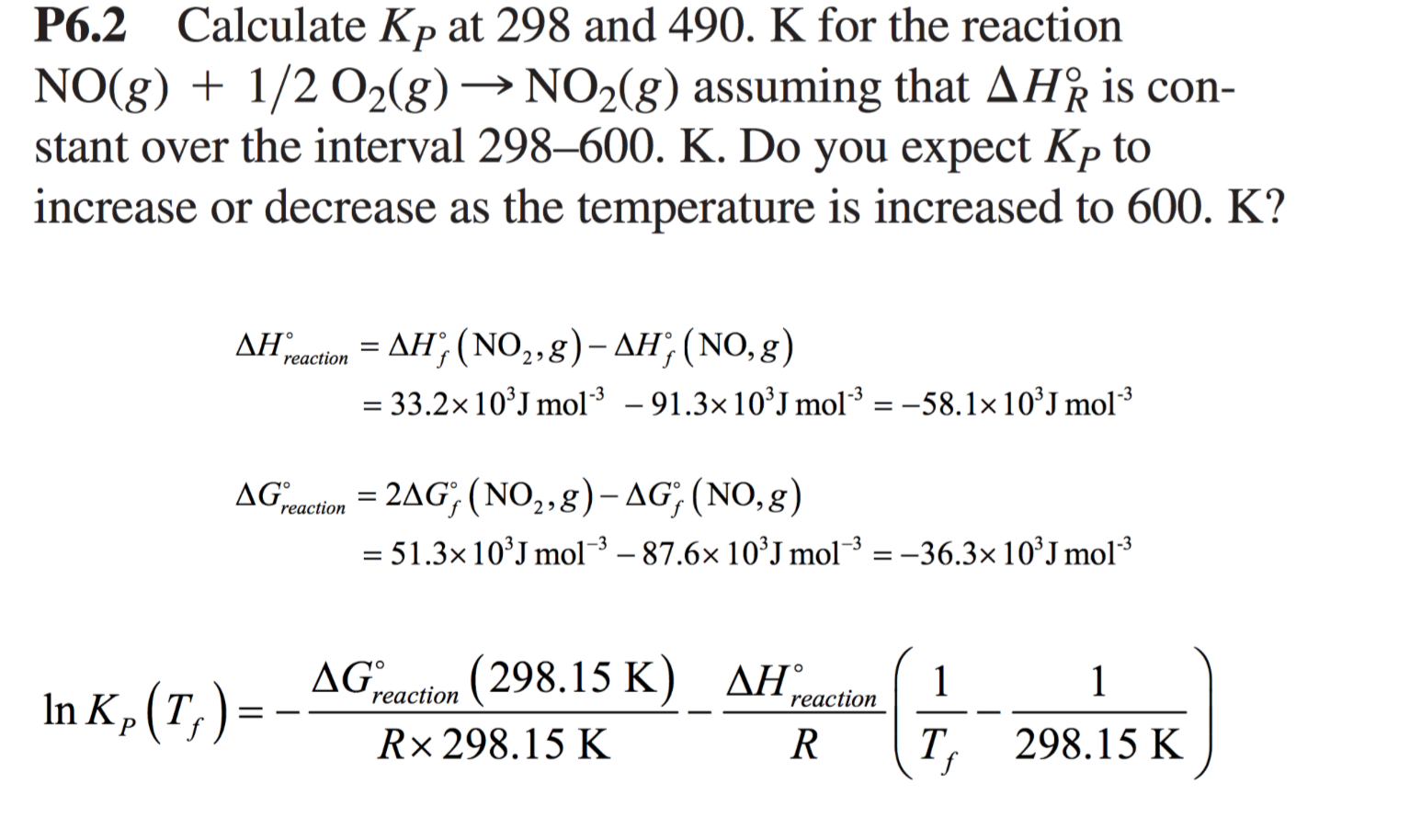
Solved Calculate Dg At 298 K For Each Reaction A 2 No G Cl2 G 2 Nocl G K 1 58 10 7 B Cu2s S O2 G 2 Cu S So2 G K 3 25 10 37
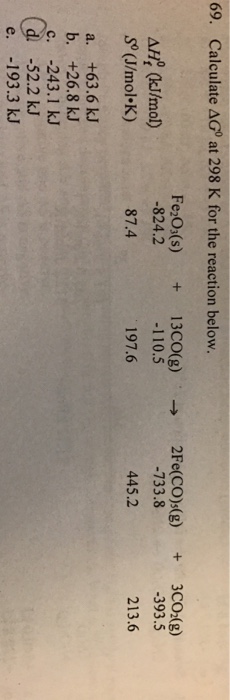
Solved Calculate Delta G Degree At 298 K For The Reaction Chegg Com

Solved Calculate Dg At 298 K For Each Reaction A 2 No G Cl2 G 2 Nocl G K 1 58 10 7 B Cu2s S O2 G 2 Cu S So2 G K 3 25 10 37
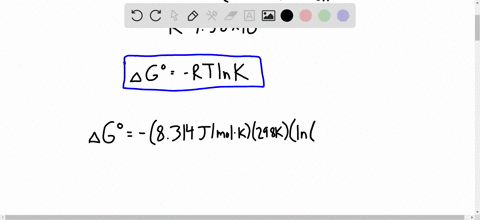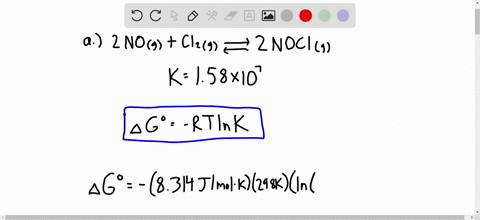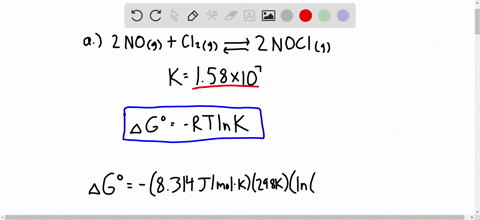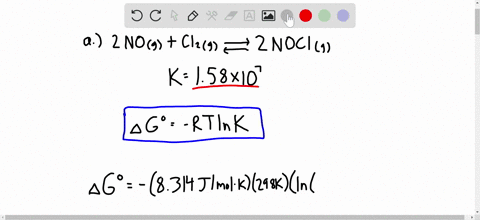
Solved Calculate Dg At 298 K For Each Reaction A 2 No G Cl2 G 2 Nocl G K 1 58 10 7 B Cu2s S O2 G 2 Cu S So2 G K 3 25 10 37
Solved In The First Question Delta G Of Reaction Is Added In Chegg Com

Innovative Chemistry Revision Notes Chem184 Innovative Chemistry For Energy And Materials Liverpool Thinkswap
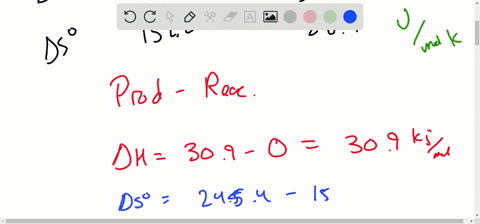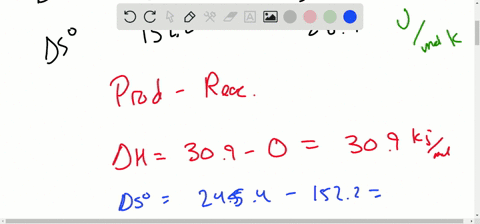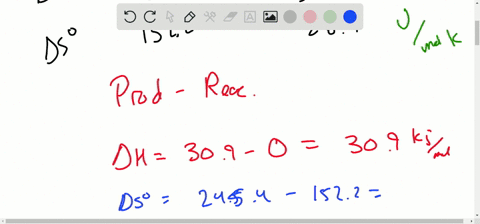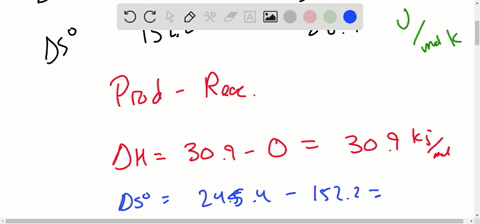
Solved Calculate Dg At 298 K For Each Reaction A 2 No G Cl2 G 2 Nocl G K 1 58 10 7 B Cu2s S O2 G 2 Cu S So2 G K 3 25 10 37
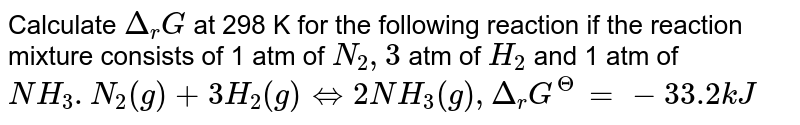
Calculate Delta R G At 298 K For The Following Reaction If The Reaction Mixture Consists Of 1 Atm Of N 2 3 Atm Of H 2 And 1 Atm Of Nh 3 N 2 G 3h 2 G Harr 2nh 3 G

Entropy Post 16 Thermodynamics Tutorials Resource Rsc Education
First Document Gsi

The Value Of D G For The Reaction At 298 K Is

Calculate Delta R G At 298 K For The Following Reaction If The Reaction Mixture Consists Of 1 Atm Of N 2 3 Atm Of H 2 And 1 Atm Of Nh 3 N 2 G 3h 2 G Harr 2nh 3 G

Solved Calculate Dg At 298 K For Each Reaction A 2 No G Cl2 G 2 Nocl G K 1 58 10 7 B Cu2s S O2 G 2 Cu S So2 G K 3 25 10 37

Calculate Dg At 298k For The Following Reaction Youtube